What is the EurOxVaP ME/CFS Collaborative?
Diagnosis & management of ME/CFS: understanding the biology & identifying therapeutic targets
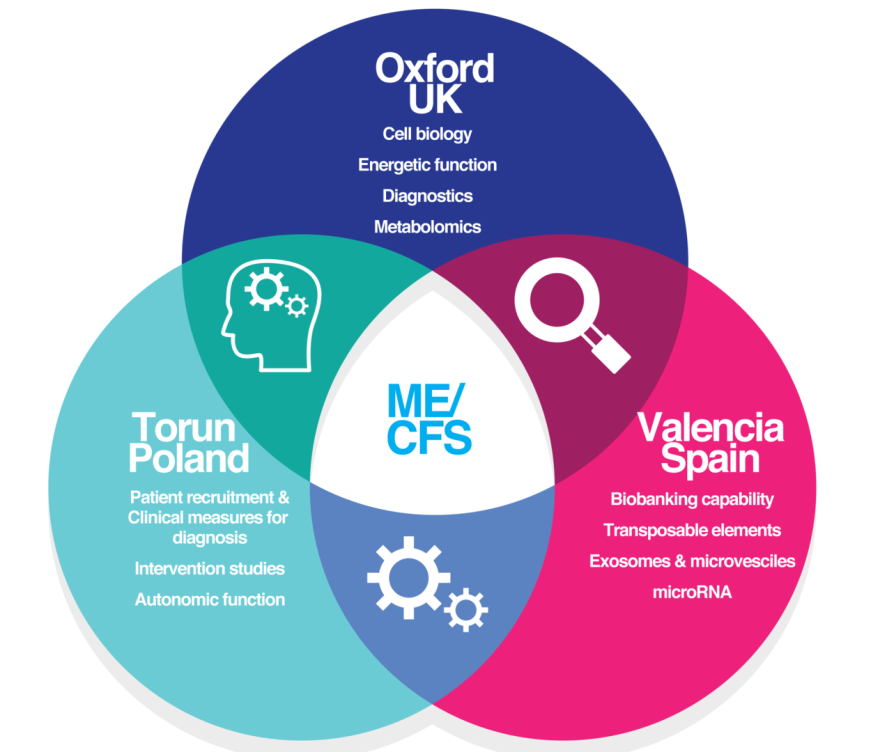
EuroOxVaP is a newly formed collaboration between the University of Oxford (UK), Nicolaus Copernicus University (Poland) and the University of Valencia (Spain) to investigate biological factors that are different between ME/CFS and other diseases that have co-morbid chronic fatigue.
Initiative 1 - planned start date: January 2021
Oxford
Testing the potential of Raman microscopy as a diagnostic test in ME/CFS .
Investigating 200 ME/CFS patients and controls, final step before a clinical trial.
Fundraising goal: £100K, testing current samples. Writing new ethics
and clinical trial design.
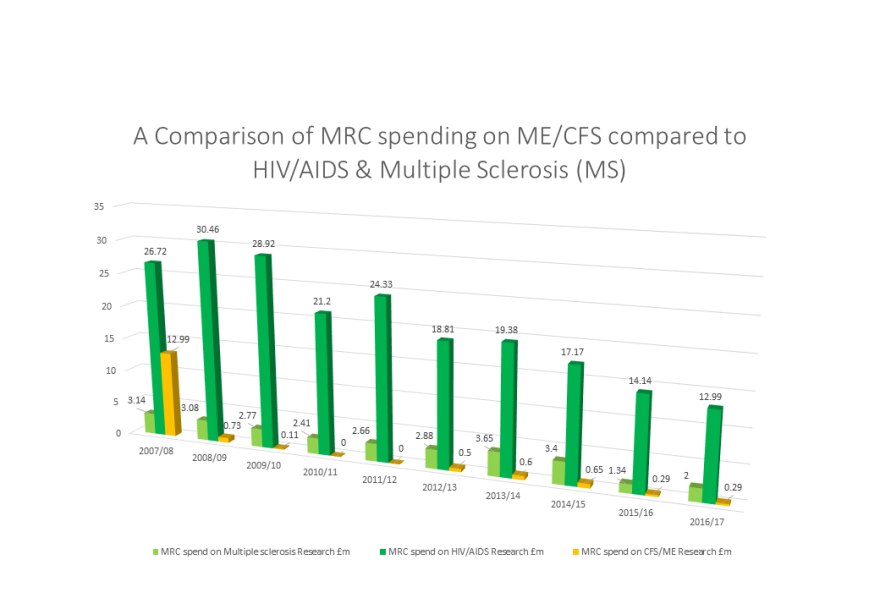
Why are we fundraising?
Multi-site collaboration
Although fatigue is a major debilitating symptom in many conditions, we still have no clear insight into its biological basis. Most fatigue Research has been carried out in ME/CFS and this, in particular the ME/CFS specific phenomenon of long lasting post – exertional exacerbation of fatigue (in addition to other symptoms), will be the primary focus with other conditions giving insight into common mechanisms associated with co-morbid chronic fatigue.
Disesase Mediators
Using this approach, we will be able to identify ME/CFS specific mechanisms, which would not be possible if only a healthy control population were to be used. Through metabolomics, How and through what means (explicitly) can why can we identify ME/CFS disease mediators) / blood samples will be collected for future biomarker discovery projects. Our recent metabolomics analysis of two ME/CFS cohorts has emphasised the importance of this approach and the benefit of using a multiple sclerosis group as a control. We will therefore be using comprehensive metabolite profiling during both fasted state and non-fasted states to control for the variance that can occur during these phases.
Interventions
Our clinical research will involve three European centres and build on existing infrastructure. Pilot treatment interventions will be undertaken in all chronic fatigue groups to help illuminate similar or disimilar biochemical and physiological pathways.
(Intervention with randomised, single blinded Isotonic (0.9%) Saline for all patients regardless of POTS/OI status) with a washout period and a latter period whereby a placebo non-isotonic drink is administered.
-------
Notably, a systematic review of the placebo response in ME/CFS was found to be low at 19.6% (HJ Cho et Al, 2005).
Additionally, as identified through metabolomics, some patients exhibit substantial elevations in the amount of glutamate (a neuro-excitotatory amino acid) present in their blood. With a decreased ratio of GABA:glutamate, compared to healthy controls, it is possible that peripheral circulating metabolites can provide an inference to brain cell metabolism. However, salivary samples would provide a superior and non-invasive means to do so.
Once funded, through Euroxtova salivary samples will be taken across all countries at baseline and after a cardiopulmonary (CPET) exercise test. Patients with elevated glutamate and or decreased GABA:gluamate profiles will be treated with the Excitatory Amino Acid Transporter 2 (EAAT2) activator, Riluzole, capable of increasing neuronal glutamate uptake.